WHY THIS MATTERS IN BRIEF
Bit by bit animal testing is slowly being replaced by new more modern methods, the FDA’s new “Humans on Chips” collaboration will accelerate its demise and make drug testing cheaper, faster and safer.
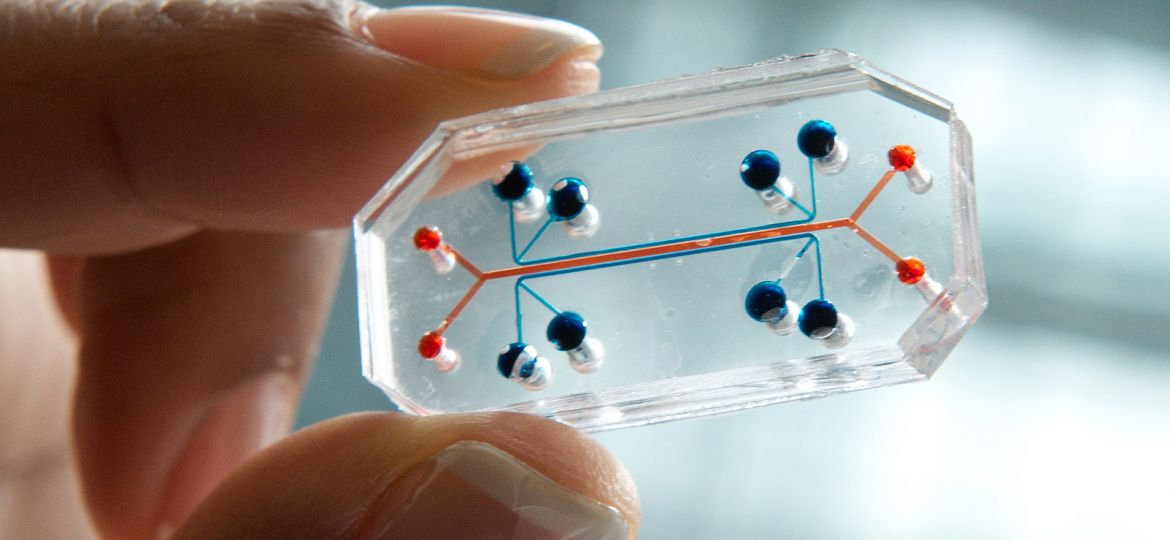
A future without animal testing has taken a step closer this week after the US Food and Drug Administration (FDA) agreed to enter into a research and development collaboration with Emulate Inc., a company that makes Organs on a Chip, a new technology that does what it says on the tin – puts human organs, such as hearts, kidneys and livers, and all their biological foibles and characteristics, onto small, cheap, disposable plastic chips.
The FDA’s ultimate goal is to replace all of today’s animal based drug and supplement testing with just a simple chip, each of which is the size of a human thumb that contain tiny channels filled with living human cells that imitate the functions of different human organs.
Furthermore, while these chips are their own technological marvels, and they can even be combined together create more complex biological systems, they’re also very cheap to make and generate results in fractions of the amount of time it takes today, speeding up, and reducing the cost of all of today’s drug testing procedures in one go. A win win win.
The gallery was not found!
For example, in Emulate’s case their Lung-on-a-Chip recreates how the human lung reacts to certain medications, and it’s also hoped that the results of the tests that use the chips will be more accurate than today’s tests that use a culture of lung cells or an animal’s lung – neither of which perfectly mirror the intricacies, or biology, of the human lung like the new chips do.
Animal testing is an unfortunate, but still critical, part of most drug development, and before a drug makes it to the FDA, the company behind it has to show its effect on animals, and, more specifically, whether it’s toxic.
Today’s procedures require scientists to run tests on different animals and then hand that data over to the FDA for analysis and then, and only then, if the FDA signs it all off, can the company start trials in humans.
The new collaboration between the FDA and Emulate will initially focus on the company’s Liver-on-a-Chip product that shows how a human liver, which is where most drugs get broken down on their way out of the body, will react to certain drugs.
While animal testing doesn’t look like it’s going to go away anytime soon the FDA’s endorsement of this new important technology is a crucial step that signals the beginning of the end for animal testing in pre-clinical research. And if it can at least help reduce the number of animals that suffer in animal testing labs then that’s only ever going to be a positive move.drug
















