WHY THIS MATTERS IN BRIEF
- Hundreds of thousands of babies are born prematurely and, one day, this breakthrough will offer parents new hope, it could also signal the beginning of humanity’s ability to create huge Star Wars like clone armies…
In February a team of researchers from the UK’s Cambridge University and New York’s Rockefeller University created the world’s first artificial womb, but it was on a small scale and, for want of a better description, was based in a jar. Now a team of researchers from the Children’s Hospital of Philadelphia (CHP) have gone one step, arguably, three steps further and built a fully functioning artificial womb in what amounts to a ziplock bag, and they’ve used it to help develop eight foetal lambs – add that to the fact that we can also create artificial human sperm and eggs are we could be heralding the beginning of the end, albeit still a long way away, of the “traditional” mother, father and child relationship. Think about it – that’s kind of scary, we’re entering the realm of sci-fi right here and right now, and we know it’ll come one day because the future always arrives. The only question in when.
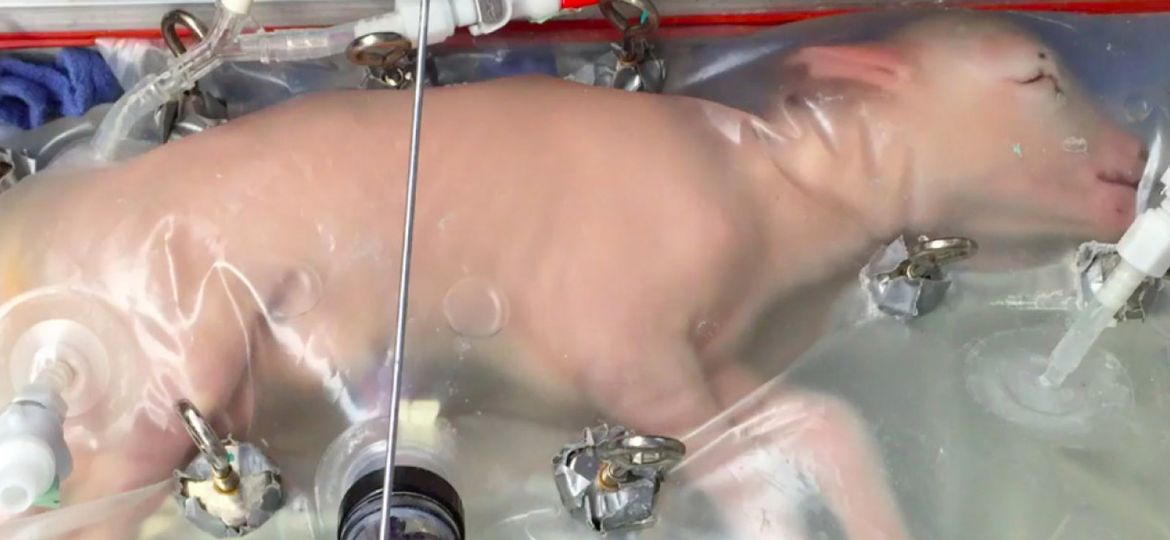
Inside the new artificial wombs, which are strewn with tubes of blood and fluid, the foetal lambs continued to develop in an almost natural way, with the obvious thoughts, and over a four week period their lungs and brains grew, they sprouted wool, opened their eyes, wriggled around, and learned to swallow. Consequently one day it’s hoped the new breakthrough device will help bring premature human babies to term outside the uterus but for now it’s only been tested on sheep.
While it’s appealing to imagine a world where artificial wombs grow babies and eliminate the health risks and stress of pregnancy experts say it’s important not to get ahead of ourselves.
“It’s complete science fiction to think that you can take an embryo and get it through the early developmental process and put it on our machine without the mother being the critical element there,” says Alan Flake, a foetal surgeon at CHP and lead author of the study which was published in the journal Nature Communications.
Instead, the point of developing an external womb, which his team calls the Biobag, is to give infants born months too early a more natural, uterus-like environment to continue developing in, Flake says.
The Biobag may not look much like a womb, but it contains the same key parts and ingredients, and that’s the important thing. The clear plastic bag that encloses the foetal lamb and protects it from the outside world, like the uterus would, contains an electrolyte solution that bathes the lamb in a similar way to the mother’s natural amniotic fluid, and a way for the foetus to circulate its blood and exchange carbon dioxide for oxygen.
The gallery was not found!
Flake hopes the Biobag will improve the care options for extremely premature infants, who have “well documented, dismal outcomes,” he says.
Premature births is one of the leading causes of death for infants, and with around ten percent of all babies in the US being born prematurely, or before 37 weeks of gestation, over 30,000 of those babies are born extremely prematurely and they require intensive support as they continue to develop outside their mothers’ bodies. The babies fortunate enough to survive delivery then require mechanical ventilation, medications, and IVs that provide nutrition and fluids, and if – and it’s a big if – they make it out of the intensive care unit then up to half of them will suffer from a host of long term health conditions such as long term developmental problems, learning problems and a myriad of other complications.
“So parents have to make critical decisions about whether to use aggressive measures to keep these babies alive, or whether to allow for less painful, comfort care,” says neonatologist Elizabeth Rogers, co-director for the Intensive Care Nursery Follow-Up Program of UCSF Benioff Children’s Hospital, who was not involved in the study, “one of the unspoken things in extreme preterm birth is that there are families who say, ‘If I had known the outcome for my baby could be this bad, I wouldn’t have chosen to put her through everything.’”
That’s why for decades scientists have been trying to develop an artificial womb that would re-create a more natural environment for a premature baby to continue to develop in – rather than an incubator. But one of the main challenges was re-creating the intricate circulatory system that connects the mother to the foetus which lets the mother’s blood flow to the baby and back, exchanging vital life giving oxygen for carbon dioxide. The blood needs to flow with just enough pressure, but an external pump can damage the baby’s heart.
To solve this problem, Flake and his colleagues created a pumpless circulatory system. They connected the foetus’s umbilical blood vessels to a new kind of oxygenator, and the blood moved smoothly through the system. Smoothly enough, in fact, that the baby’s heartbeat was sufficient to power blood flow without another pump.
The next problem to solve was the risk for infections, which premature infants in open incubators face in the neonatal intensive care unit, or NICU. That’s where the bag and the artificial amniotic fluid comes in. The fluid flows in and out of the bag just like it would in a uterus, removing waste, shielding the infant from infectious germs in the hospital, and keeping the foetus’s developing lungs filled with fluid.
Flake and his colleagues tested the setup for up to four weeks on eight foetal lambs that were 105 to 120 days into pregnancy, which is about equivalent to human infants at 22 to 24 weeks of gestation. After the four weeks were up, they were switched onto a regular ventilator like a premature baby in a NICU.
The lambs’ health on the ventilator appeared nearly as good as a lamb the same age that had just been delivered by caesarean section. Then, the lambs were removed from the ventilator and all but one, which was developed enough to breathe on its own, were euthanised so the researchers could examine their organs. Their lungs and brains — the organ systems that are most vulnerable to damage in premature infants — looked uninjured and as developed as they should be in a lamb that grew in a mother.
Of course, lambs aren’t humans though, and their brains develop at a somewhat different pace. The authors acknowledge that it’s going to take more research into the science and safety of this device before it can be used on human babies – and then there are the ethical and regulatory limitations. They’ve already started testing it on human-sized lambs that were put in the Biobags earlier in pregnancy, and they’re monitoring the few lambs that survived after being taken off the ventilator to look for long term problems, however, so far, the lambs seem pretty healthy.
“I think it’s realistic to think about three years for first-in-human trials,” Flake says.
“It’s so interesting, and it’s really innovative,” Rogers says, “to be able to continue to develop in an artificial environment can reduce the many problems caused by simply being born too early.”
“I’m still blown away, whenever I’m down looking at our lambs,” he says, “I think it’s just an amazing thing to sit there and watch the foetus on this support acting like it normally acts in the womb… It’s a really awe-inspiring endeavour to be able to continue normal gestation outside of the mom.”
The research continues.
















